
<
Serge77 - My Rocket Workshop >Solubility of KNO3 and Other Salts
in Molten Sorbitol
Starting materials:
KNO3: reagent grade "pure for analysis". Moisture content 0.1-0.2%.
NaCl: table salt. Moisture content 0.1-0.2%.
NaNO3: reagent grade "pure for analysis". Moisture content 0.1-0.2%.
Sorbitol: pharmaceutical grade. Moisture content 0.2-0.3%.

General method:
sorbitol (10.00 g) was melted, resulting milky-white liquid was heated until it become transparent (170-180°C), cooled to 80°C, then KNO3 or other salt was added. Resulting mixture was slowly heated on magnetic stirrer until complete dissolution observed (T1). Heating rate near the dissolution point was 1 degree at 5-10 min.
Upon cooling all solutions become cloudy at some point (T2) due to beginning of precipitation of very small crystals.
KNO3
|
Sorbitol |
KNO3 |
T1 (°C) |
T2 (°C) |
|
10.00 g |
2.00 g |
110 |
80 |
|
10.00 g |
3.00 g |
135 |
120 |
|
10.00 g |
4.00 g |
160 |
155 |
|
10.00 g |
5.00 g |
180 |
175 |
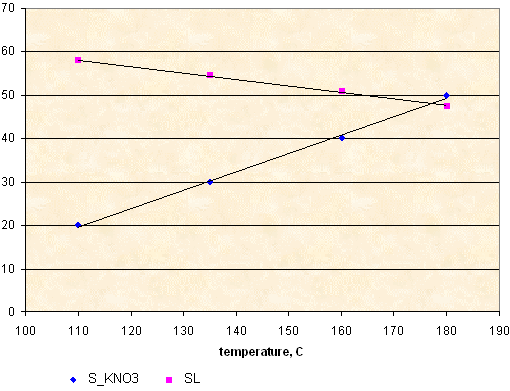
S_KNO3 - solubility, g KNO3 per 100 g of sorbitol
SL - solids loading of molten KNSB(65-35) propellant, calculated according to solubility data.
S_KNO3 = 0.424 * T - 27.1
SL = 74.5 - 0.149 * T
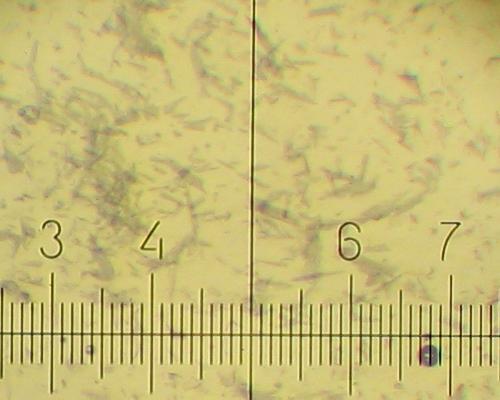
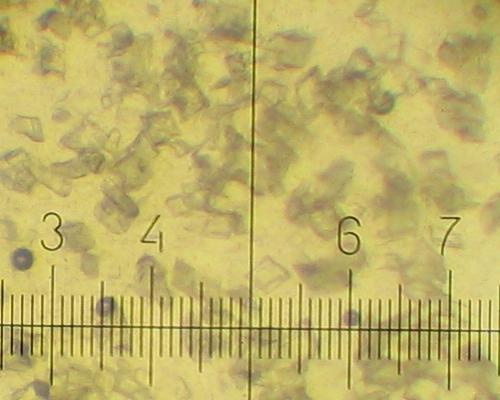
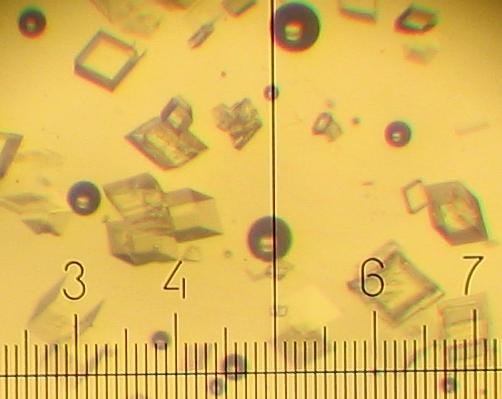
Different types of KNO3 crystals grow during cooling of the solution and further standing at room temperature.
20% KNO3 solution deposits fine needles 30-90 micron long and less than 5 micron wide. (One small division on the scale is equal to 14 micron).
All other solutions deposit 20-40 micron prisms during cooling:
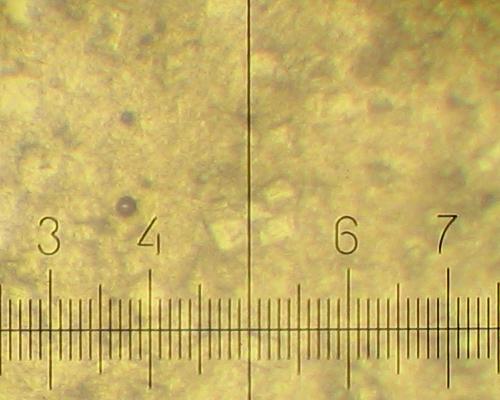
After standing at room temperature a plenty of very fine crystals (5-15 micron) grow in addition:
NaCl
|
Sorbitol |
NaCl |
T1 (°C) |
|
10.00 g |
0.25 g |
130 |
|
10.00 g |
0.50 g |
165 |
|
10.00 g |
~0.95 g (*) |
240 |
(*) In this experiment 1.00 g of NaCl was loaded, but at 240°C sorbitol decomposition started, so heating was terminated. About 0.05 g of NaCl remained undissolved.
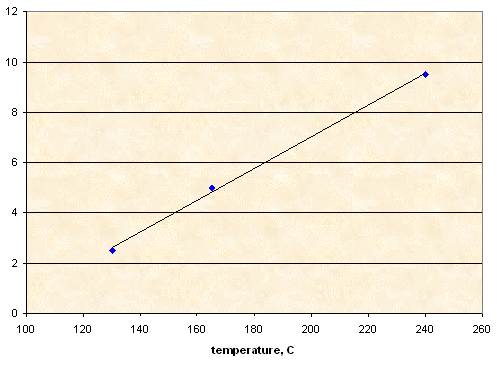
Solubility, g NaCl per 100 g of sorbitol
NaNO3
|
Sorbitol |
NaNO3 |
T1 (°C) |
T2 (°C) |
|
10.00 g |
3.00 g |
125 |
95 |
|
10.00 g |
4.00 g |
150 |
140 |
|
10.00 g |
5.00 g |
175 |
165 |
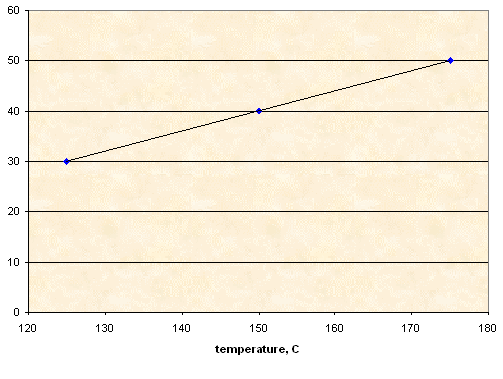
Solubility, g NaNO3 per 100 g of sorbitol
Mixture KNO3-NaNO3 1:1
|
Sorbitol |
KNO3+NaNO3 |
T1 (°C) |
|
10.00 g |
4.00 g |
110 |
|
10.00 g |
6.00 g |
130 |
|
10.00 g |
8.00 g |
145 |
|
10.00 g |
10.00 g |
160 |
|
10.00 g |
12.00 g |
170 |
|
10.00 g |
14.00 g |
175 |
|
10.00 g |
16.00 g |
178 |
|
10.00 g |
20.00 g |
180 |
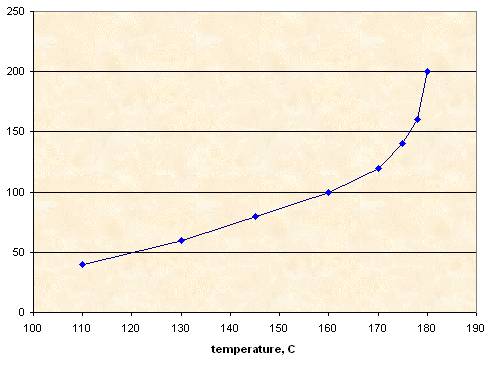
Solubility, g KNO3+NaNO3 per 100 g of sorbitol
I have posted about this interesting system on AROCKET in 2002 under the name Liquid Candy. Burn rate of propellant KNO3-NaNO3-sorbitol 1:1:1 is 1 mm/s.
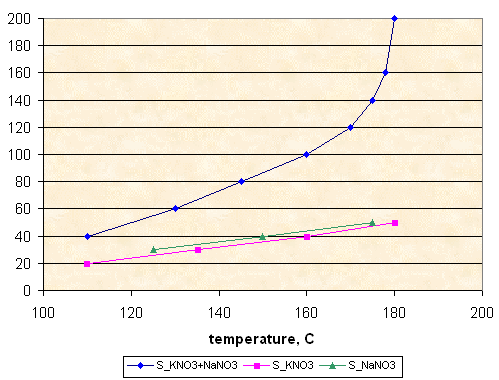
Solubility, g per 100 g of sorbitol
This effect of increasing solubility can be used for lowering of propellant viscosity. For example, molten propellant KNO3-NaNO3-sorbitol 60-5-35 is less viscous at 120°C, than KNO3-sorbitol 65-35. Composition KNO3-NaNO3-sorbitol 55-10-35 is even less viscous. Composition KNO3-NaNO3-sorbitol 35-30-35 is very runny at 120°C.
Molten propellants KNO3-NaNO3-sorbitol-sucrose 35-30-15-20 (at 120°C) and 35-30-5-30 (at 140°C) are much more fluid, than standard KNSB, they can be easily poured in paper tube 17 mm ID directly from the wide pan. I will report about these propellants later.
17.01.2006 Serge77